You are using a browser that is not supported by this site. The site will not function properly. Please switch to the latest version of a supported browser such as Chrome, Safari, Edge, or Firefox to use this site.
CADD-Legacy® ambulatory infusion pump

LIMITED SUPPLY AVAILABLE*
*Contact the Specialty Pharmacy with questions.
CADD-Legacy® ambulatory infusion pump—for continuous delivery of IV Remodulin
The CADD-Legacy® ambulatory infusion pump is proven for continuous IV delivery of Remodulin and other medicines.
Benefits of CADD-Legacy® ambulatory infusion pump1
- Easy-to-read display that provides information for programming adjustments and troubleshooting
- Reliability and durability can mean less downtime and fewer interruptions of therapy
- Appropriate for patients who are titrating or on a stable dose of Remodulin
- Up to 2 days between pump refills2
- Requires patient to dilute Remodulin with diluent and then fill the pump in a sterile field for each refill3
- Option to have a 7-day supply of Remodulin shipped to patient’s home
Download the Remodulin Referral Form to specify that your patient receives the ambulatory IV infusion pump for Remodulin.
Mixing Options
On-demand mix at each cassette change (every 2 days)
Premix a 14-day supply with a simple premixing schedule
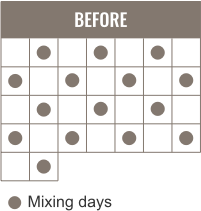
Simple premixing schedule

SPmix delivers weekly shipments to eligible patients
Before introducing the topic of premixing with a patient:
- Carefully consider your patient’s dosing schedule, mixing proficiency, and diluent
- Be sure to contact the Specialty Pharmacy (SP). Specialty Pharmacies can answer any questions they may have about premixing and supply the necessary diluent needed for 14-day premixing. Be sure to inform the SP if you deem a patient appropriate for premixing at home
Remind patients to continue to change the reservoir at least every 2 days.
Be sure to share the following information with your patients
- 14-day premixing should be done only with high-pH glycine diluent. Remodulin cannot be stored for 14 days when mixed with sterile water for injection or 0.9% sodium chloride injection (only 4 hours at room temperature or 24 hours refrigerated)3
- Reservoir tubing should be capped before storage
- Do not use reservoirs that have been stored for more than 14 days
- Use extension tubing with a filter
- The CADD-MS® 3 ambulatory infusion pump should not be used for premixing
IV=intravenous.
Remodulin SPmix Program
The Remodulin SPmix Program delivers up to 7 days of premixed IV Remodulin and all necessary supplies to your patients’ homes each week.
Remodulin® (treprostinil) Injection
Important Safety Information for Remodulin
Warnings and Precautions
- Chronic intravenous (IV) infusions of Remodulin delivered using an external infusion pump with an indwelling central venous catheter are associated with the risk of blood stream infections (BSIs) and sepsis, which may be fatal. Therefore, continuous subcutaneous (SC) infusion is the preferred mode of administration.
- Avoid abrupt withdrawal or sudden large reductions in dosage of Remodulin, which may result in worsening of PAH symptoms.
- Titrate slowly in patients with hepatic insufficiency, because such patients will likely be exposed to greater systemic concentrations relative to patients with normal hepatic function.
- Remodulin is a pulmonary and systemic vasodilator. In patients with low systemic arterial pressure, treatment with Remodulin may produce symptomatic hypotension.
- Remodulin inhibits platelet aggregation and increases the risk of bleeding.
Adverse Reactions
- In clinical studies of SC Remodulin infusion, the most common adverse events reported were infusion site pain and infusion site reaction (redness, swelling, and rash). These symptoms were sometimes severe and sometimes required treatment with narcotics or discontinuation of Remodulin. The IV infusion of Remodulin with an external infusion pump has been associated with a risk of blood stream infections, arm swelling, paresthesias, hematoma, and pain. Other common adverse events (≥3% more than placebo) seen with either SC or IV Remodulin were headache (27% vs. 23%), diarrhea (25% vs. 16%), nausea (22% vs. 18%), rash (14% vs. 11%), jaw pain (13% vs. 5%), vasodilatation (11% vs. 5%), edema (9% vs. 3%), and hypotension (4% vs. 2%).
Drug Interactions
- Remodulin dosage adjustment may be necessary if inhibitors or inducers of CYP2C8 are added or withdrawn.
Specific Populations
- In patients with mild or moderate hepatic insufficiency, decrease the initial dose of Remodulin to 0.625 ng/kg/min of ideal body weight, and monitor closely. Remodulin has not been studied in patients with severe hepatic insufficiency.
- Safety and effectiveness of Remodulin in pediatric patients have not been established.
- It is unknown if geriatric patients respond differently than younger patients. Caution should be used when selecting a dose for geriatric patients.
- There are no adequate and well-controlled studies with Remodulin in pregnant women. It is not known whether treprostinil is excreted in human milk or if it affects the breastfed infant or milk production.
Indication
Remodulin is a prostacyclin vasodilator indicated for the treatment of pulmonary arterial hypertension (PAH; WHO Group 1) to diminish symptoms associated with exercise. Studies establishing effectiveness included patients with NYHA Functional Class II-IV symptoms and etiologies of idiopathic or heritable PAH (58%), PAH associated with congenital systemic-to-pulmonary shunts (23%), or PAH associated with connective tissue diseases (19%).
In patients with PAH requiring transition from epoprostenol, Remodulin is indicated to diminish the rate of clinical deterioration. Consider the risks and benefits of each drug prior to transition.
REMISIhcpFEB2025
Please see accompanying Full Prescribing Information for Remodulin.
For additional information, visit www.RemodulinPro.com or call Customer Service at 1-844-UNITHER (1-844-864-8437).
For additional information, visit www.RemodulinPro.com or call Customer Service at 1-844-UNITHER (1-844-864-8437).
Remodulin® (treprostinil) Injection
Important Safety Information for Remodulin
Warnings and Precautions
- Chronic intravenous (IV) infusions of Remodulin delivered using an external infusion pump with an indwelling central venous catheter are associated with the risk of blood stream infections (BSIs) and sepsis, which may be fatal. Therefore, continuous subcutaneous (SC) infusion is the preferred mode of administration.
- Avoid abrupt withdrawal or sudden large reductions in dosage of Remodulin, which may result in worsening of PAH symptoms.
- Titrate slowly in patients with hepatic insufficiency, because such patients will likely be exposed to greater systemic concentrations relative to patients with normal hepatic function.
- Remodulin is a pulmonary and systemic vasodilator. In patients with low systemic arterial pressure, treatment with Remodulin may produce symptomatic hypotension.
- Remodulin inhibits platelet aggregation and increases the risk of bleeding.
Adverse Reactions
- In clinical studies of SC Remodulin infusion, the most common adverse events reported were infusion site pain and infusion site reaction (redness, swelling, and rash). These symptoms were sometimes severe and sometimes required treatment with narcotics or discontinuation of Remodulin. The IV infusion of Remodulin with an external infusion pump has been associated with a risk of blood stream infections, arm swelling, paresthesias, hematoma, and pain. Other common adverse events (≥3% more than placebo) seen with either SC or IV Remodulin were headache (27% vs. 23%), diarrhea (25% vs. 16%), nausea (22% vs. 18%), rash (14% vs. 11%), jaw pain (13% vs. 5%), vasodilatation (11% vs. 5%), edema (9% vs. 3%), and hypotension (4% vs. 2%).
Drug Interactions
- Remodulin dosage adjustment may be necessary if inhibitors or inducers of CYP2C8 are added or withdrawn.
Specific Populations
- In patients with mild or moderate hepatic insufficiency, decrease the initial dose of Remodulin to 0.625 ng/kg/min of ideal body weight, and monitor closely. Remodulin has not been studied in patients with severe hepatic insufficiency.
- Safety and effectiveness of Remodulin in pediatric patients have not been established.
- It is unknown if geriatric patients respond differently than younger patients. Caution should be used when selecting a dose for geriatric patients.
- There are no adequate and well-controlled studies with Remodulin in pregnant women. It is not known whether treprostinil is excreted in human milk or if it affects the breastfed infant or milk production.
Indication
Remodulin is a prostacyclin vasodilator indicated for the treatment of pulmonary arterial hypertension (PAH; WHO Group 1) to diminish symptoms associated with exercise. Studies establishing effectiveness included patients with NYHA Functional Class II-IV symptoms and etiologies of idiopathic or heritable PAH (58%), PAH associated with congenital systemic-to-pulmonary shunts (23%), or PAH associated with connective tissue diseases (19%).
In patients with PAH requiring transition from epoprostenol, Remodulin is indicated to diminish the rate of clinical deterioration. Consider the risks and benefits of each drug prior to transition.
REMISIhcpFEB2025
Please see accompanying Full Prescribing Information for Remodulin.
For additional information, visit www.RemodulinPro.com or call Customer Service at 1-844-UNITHER (1-844-864-8437).
References: 1. Operator’s Manual: CADD-Legacy™ 1 Ambulatory Infusion Pump. St. Paul, MN: Sims Deltec, Inc; 2000. 2. Benza RL, Tapson VF, Gomberg-Maitland M, et al. One-year experience with intravenous treprostinil for pulmonary arterial hypertension. J Heart Lung Transplant. 2013;32(9):889-896. 3. Remodulin [package insert]. Research Triangle Park, NC: United Therapeutics Corporation; 2023.
